Pitting Corrosion
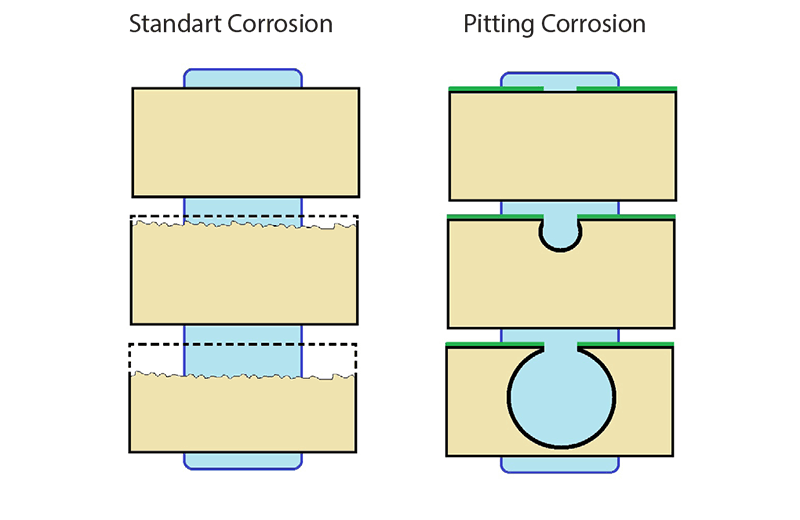
Pitting corrosion is a localized form of corrosion by which cavities or “holes” are produced in the material. Pitting is considered to be more dangerous than uniform corrosion damage because it is more difficult to detect, predict and design against. Corrosion products often cover the pits.
Boiler Deaerators
Boiler deaerators are used to remove oxygen and other gases from the water that feeds into boilers that generate steam.
Water temperature must be more than 85 degree celcuis to remove oxygen and the other gases from the water. At 85 degree celcuis deaeration starts. At 105 degree celcuis 95% of the oxygen in the water is dissolved.
Deaerators remove the gases that attach to the metallic components of the steam system and cause corrosion by forming oxides, or rust. Oxygen and carbon dioxide are responsible for corrosion. There are two types of boiler deaerators: Tank model or compact deaerators.

When water passes from the feed water tank into the boiler deaerator, it enters through the inlet water connection. The water flows through a heating and venting section that is filled with steam. The water temperature rises, which releases most of the undissolved gases in it including oxygen and carbon dioxide. When the water flows through the deaerator, it passes to the scrubber section. It is in this section where the last step of deaeration takes place because it scrubs the water with steam that is free of oxygen. Then, the steam goes through a stainless steel spray valve that breaks down the high-velocity steam into a fine mist. The deaerated water flows over to the storage compartment and is ready for use by the boiler while the gases vent to the atmosphere. By virtually eliminating the amount of dissolved oxygen and carbon dioxide in the feed water, boiler deaerators help lower operating costs and improve steam quality for facilities.

